
The Chelation Relation: The Art of a Good Chelate
Hi Everyone! Dr. NPK here, at it yet again. Today’s blog topic is about chelation: a very chemistry-ish topic! Chelation is a very important chemical principle that is important in a variety of industries, including cleaners, water purification, and oil & gas. BUT MOST IMPORTANTLY: chelation is important in growing cannabis 😊.
The Definition of Chelation
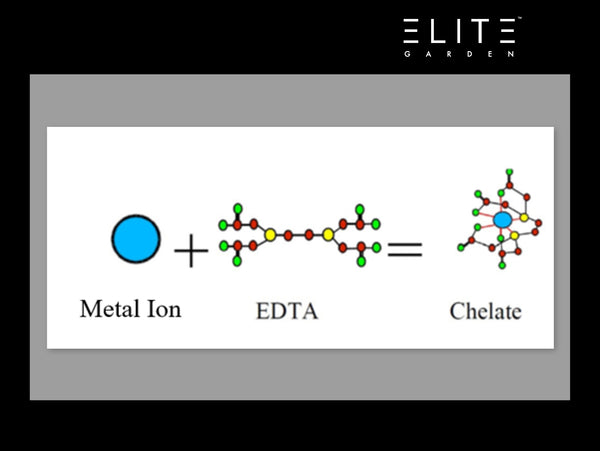
Chelation (pronounced key-lay-shun) is defined as: “the process of chelating” …thanks Merriam-Webster. Well anyway, the act of chelating is a type of bonding between a metal ion and a ligand (ligand = a type of molecule that has an affinity for the metal ion). An analogy of this would be to imagine your significant other in a crowded room…if he/she was asked to give ONE person a hug in the room, (hopefully it’s you), that’s how ligand/chelants work. These chelating agents have different affinities based on size and ion type (e.g., calcium has certain attractions to chelants that iron does not). The generic equation for a chelant is: M + L = ML (M = metal, L = ligand). Once the ligand binds to the metal ion, it is usually chemically unfavorable for the ligand to separate from the metal (meaning the reaction is not reversible…once you make “ML”…it will be really hard to split them up!). Imagine the chelating agent “biting” the metal and holding on….
The Chelation Connection to Cannabis (CCC)
It’s cool that chelation is an important principle in a lot of industries…but why is it important in cannabis? If you recall from above, I had mentioned that chelation is important for metal ions. Macronutrients like potassium, sulfur, and nitrogen do not require chelation to be taken up by the plant. Cannabis plants (and all crops) need a variety of nutrients, many of which are metal ions. These include most of the micronutrients found in Elite Base Nutrient A, like iron (Fe), manganese (Mn), zinc (Zn), and copper (Cu). Unfortunately, these highly charged species get “caught up” by other ions present in the tank (or soil). The scientific term for this is “double displacement reaction.” These positively charged metals mentioned above react with the negative species found in your sump (such as sulfate, or phosphate), and create an INSOLUBLE metal mixture! Insoluble mixture = not taken up as easily by the plant = micronutrient deficiency! In the case of iron, the heavily oxygenated environment causes the iron to be oxidized, making it water insoluble and in a plant-unusable form. By using a chelated metal, it assures that the chemical identity of the micronutrient stays the same (i.e., does not interact with the negative species found in your sump), ensuring that the micronutrient is delivered in plant-usable form.
EDTA: The Best Bodyguard of Them All
There are a variety of chelant chemistries available; but one stands head and shoulders among the rest. I am referring to EDTA (ethylenediaminetetraacetic acid). EDTA is an attractive chelating agent because it is cost effective and has broad-pH stability; when I say pH-stable, it means that the chelated complex of the metal+chelant does not separate at the specified pH. EDTA is the chelant of choice in hydroponics for most applications; the one exception is really, really, hard water with a slightly alkaline pH (>7.0). If your water is this hard (my condolences), consider pH adjusting the water prior hitting it with nutrients. If you are seeing a super red “blood-like” color to your nutrient mix, it is likely oxidized iron that is not plant-usable. If you are running your res at a normal pH range, EDTA should be good to go. There are some naysayers that say EDTA is harmful; I disagree with those people. EDTA is a common chelating agent in the pharmaceutical industry (Wax, P. J. Med. Toxicol. 2013, 9, 303–307) and its metabolism in chickens (Darwish, N. M.; Kratzer, J. Nutr. 1965, 86, 187–192) and cells (Kluner, T.; et al. Applied Microbiology and Biotechnology 1998, 49, 194–201) has been studied. I can buy a bottle of calcium EDTA from any grocery store…so it feels pretty safe to me.

Closing Thoughts
Chelation is an important chemical principle in hydroponic growing. Fortunately, for most growers EDTA is the best solution for your chelated micros due to its availability and general stability. If your water is outside the normal pH range for EDTA, consider pre-treating your water with pH up/down to get to a normal range prior to treating with nutrients.




